Effects of Budesonide Plus Vitamin AD on Children with Bronchial Asthma and the Effect on Serum IgE and C-reactive Protein
Yan Wang1#, Yongpan Tan2#, Lian Zhang3, Lina Zheng1, Liping Han1, Juan Xie1, Yao Cui1, Ming Zhang1, Xiaoyan An1*
1Neonatology Department, Shijiazhuang Fourth Hospital, Shijiazhuang, Hebei Province, China
2Ultrasound Department, Shijiazhuang Fourth Hospital, Shijiazhuang, Hebei Province, China
3Neonatology Department, Dingzhou People’s Hospital, Dingzhou, Hebei Province, China
#Both authors contributed equally to this manuscript.
*Correspondence to: Xiaoyan An, Neonatology Department, Shijiazhuang Fourth Hospital, No 91 Xueyuan Road, High-Tech Development Zone, Shijiazhuang 050011, Hebei Province, China; Email:18501756077@163.com
Objective: To investigate the efficacy of budesonide plus vitamin AD in children with bronchial asthma and the effect on serum IgE and C-reactive protein (CRP).
Methods: In this retrospective analysis, 80 children with bronchial asthma treated in our hospital from October 2018 to October 2020 were recruited and assigned 1:1 to receive either vitamin AD (control group) or budesonide plus vitamin AD (study group), with 40 cases in each group.
Results: Budesonide potentiated the treatment efficacy of vitamin AD by 17.5% (P<0.05). Patients receiving the combined therapy exhibited more reduction in the serum concentrations of IgE and CRP versus those with monotherapy of vitamin AD (P<0.05). Patients in the study group had lower bronchial asthma symptom scores than those in the control group, suggesting more symptom mitigation produced by budesonide plus vitamin AD (P<0.05). Budesonide plus vitamin AD offered better lung function restoration for patients versus vitamin AD alone, as evinced by the superior lung function indices (P<0.05). Patients with budesonide plus vitamin AD experienced a shorter duration of symptoms versus controls (P<0.05).
Conclusion: Budesonide plus vitamin AD exerts a promising clinical efficacy in treating children with bronchial asthma.
Keywords: budesonide, vitamin AD, bronchial asthma, clinical effect, IgE, C-reactive protein
1 INTRODUCTION
There are around 300 million asthma patients worldwide and nearly 30 million in China, and its prevalence has been on the rise. Bronchial asthma is a chronic non-specific inflammatory disease of the airway characterised by increased airway reactivity, and it may progress to chronic obstructive pulmonary disease or even heart failure in severe cases. Hughes et al.[1] found that bronchial asthma poses heavy psychological pressure on children and their families and may thus compromise the treatment effects. Bronchial asthma mostly manifests as coughing, chest tightness, and shortness of breath, which exacerbate in the event of ineffective interventions.
Currently, inhalation type β2 receptor agonists, anticholinergics, and glucocorticoids are clinically preferred agents for acute bronchial asthma attacks[2,3]. The inhaled glucocorticoid budesonide provides significant symptom mitigation for acute bronchial asthma and improves the pulmonary function of patients[4,5]. It has been reported that budesonide combined with vitamin AD provides significant relief of clinical symptoms for pateints with bronchial asthma. It has been found that vitamin AD could effectively regulate the T-lymphocyte subsets in children with asthma. Bacterial lysis, a new type of immunomodulator, can strengthen the patient's immune protection and effectively prevent bronchitis and recurrent infections of the respiratory tract[6].
Traditional Chinese medicine has a long history in the management of asthma with clinical benefits of few side effects and high safety. Many clinical studies have reported promising disease remission in patients with asthma.
The current research was to explore the efficiency of budesonide with vitamin AD in children with bronchial asthma and the impact on serum IgE and C-reactive protein (CRP).
2 MATERIALS AND METHODS
2.1 Baseline Patient Profiles
In this retrospective analysis, 80 children with bronchial asthma treated in our hospital from October 2018 to October 2020 were recruited and assigned 1:1 to receive either vitamin AD (control group) or budesonide plus vitamin AD (study group). Randomization was performed with an online web-based randomization tool (http://www.randomizer.org/). To hide allocation, the randomization procedure and assignment were carried out by a research assistant independent of the screening or evaluation of the subjects.
The original sample size calculation estimated an expected sample size of 40 to determine a 3-point difference between groups in a 2-sided significance test with a power of 0.8 and an alpha error level of 0.05.
The trial was undertaken per the Good Clinical Practice guidelines by the International Council for Harmonisation. The protocol was approved by the institutional review boards or independent ethics committees at each site (Approval NO. ML-PO20181018). All patients provided written informed consent per the Declaration of Helsinki principles. An independent data monitoring committee monitored safety and efficacy data.
2.2 Inclusion and Exclusion Criteria
2.2.1 Inclusion Criteria
(1) Patients with clinical symptoms such as wheezing, cough, and shortness of breath. (2) Who met the diagnostic criteria of Guidelines for the Diagnosis and Prevention of Bronchial Asthma in Children[7]. (3) Complete clinical data and good treatment compliance. (4) The study was ratified by the hospital ethics committee, and patients and their families signed the informed consent after having a full understanding of the study. (5) Without immune boosters. (6) Who had no history of immunosuppressant use and no history of infectious disease within 1 month prior to this study.
2.2.2 Exclusion Criteria
(1) Children with allergic rhinitis and sinusitis. (2) Who have been treated with other immunomodulators within 1 month. (3) Primary immune deficiency and congenital heart disease. (4) Major organ disease. (5) Pneumonia, malnutrition, diarrhoea. (6) Recent treatment with β2 receptor agonists and hormone drugs. (7) Drug allergy. (8) Hyperthyroidism.
2.3 Methods
All children were managed according to the conventional treatment for acute attack in the Guidelines for the Diagnosis and Prevention of Bronchial Asthma in Children, including cough and phlegm relieving, sedation, oxygen inhalation and Maintenance of water-electrolyte balance. Both groups were given vitamin AD drops (Approval No. H20057262, Nanjing Haijing Pharmaceutical Co., Ltd.), 1 capsule daily. The patients in the study group received 0.2mg of budesonide suspension (Approval No.H20140475, AstraZeneca, specification: 2mL/1mg) for aerosol inhalation treatment, twice daily.
The two groups supplemented their respective treatments with Yiqi Wenyang Huwei Decoction. The ingredients of the decoction included Sheng Huangshi 30g, Baishu 15g, Fangfeng 15g, Guizhi 10g, Baishao 10g, 3 slice Ginger, 6 Dazao, Zhi Gancao 6g, Xianmao 10g and Xian Lingpi 15g. The herbs were decocted with 400mL of water to obtain 200mL of filtrate, which was administered daily. The herb and decoction were provided by our outpatient Chinese medicine department.
2.4 Indicator Examination
Totally 3mL morning fasting venous blood was collected from the children and centrifuged 10min at 3000 r/min to isolate the serum, followed by the determination of Serum IgE and CRP levels using the ELISA kit supplied by Shanghai Enzyme-linked Biotechnology Co., Ltd. The test procedure was carried out as per the instructions of the kit and the test results were provided by the laboratory of our hospital.
2.5 Evaluation Criteria
2.5.1 Clinical Efficacy Evaluation
Cured: complete disappearance of clinical symptoms and normal body temperature. Markedly effective: most of the clinical symptoms disappear, with normal body temperature. Effective: the clinical symptoms are relieved and the body temperature is moderately reduced. Ineffective: clinical symptoms and body temperature show no changes or even aggravation. Total efficacy = (cured + markedly effective + effective) / total cases×100%.
2.5.2 Bronchial Asthma Symptom Scores
Daytime symptom score: 0 indicates no clinical symptoms; 1 indicates one transient asthma symptom; 2 indicates at least 2 episodes of transient asthma symptom; 3 indicates the patient has asthma symptoms most of the time, but daily activities are unaffected; 4 indicates frequent asthma symptoms that affect daily activities; 5 indicates severe symptoms that prevent daily activities.
Night symptom score: 0 indicates no clinical symptoms; 1 indicates one awakening due to asthma symptoms; 2 indicates 2 or more times of awakenings; 3 indicates more frequent awakenings resulting in sleeplessness; 4 indicates serious symptoms that prevent sleeping.
2.5.3 Lung Function Indexes
Peak expiratory flow (PEF), forced vital capacity (FVC), and forced expiratory volume in one second (FEV1) were determined by a pulmonary function analyzer.
2.6 Statistical Analysis
If the parameter beta is either a difference of means, a log odds ratio, or a log hazard ratio, then it is reasonable to assume that b is unbiased and normally distributed. SPSS20.0 was used for data analysis and GraphPad Prism 7 (GraphPad Software, San Diego, USA) to plot the graphics. The count data were analyzed by Chi-square test and expressed by n (%). The measurement data were analyzed by t-test and expressed by mean±SD. Differences were statistically significant when P<0.05.
3 RESULTS
3.1 Baseline Patient Profiles
In the control group, there were 21 males and 19 females, aged 5-12 (8.58±1.53) years. In the study group, there were 26 males and 14 females, aged 5-13 (8.19±1.75) years. The two groups were well balanced in baseline patient profiles (all P>0.05) (Table 1).
Table 1. General Data
|
Study Group |
Control Group |
χ2/t |
P |
n |
40 |
40 |
|
|
Age (years old) |
1.061 |
0.292 |
||
Gender (n) |
|
|
1.289 |
0.256 |
Male |
21 |
26 |
|
|
Female |
19 |
14 |
|
|
Course of acute attack (days) |
1.444 |
0.153 |
||
Course of bronchial Asthma (months) |
1.340 |
0.184 |
||
Severity of illness (n) |
|
|
0.833 |
0.361 |
Mild |
26 |
22 |
|
|
Moderate |
14 |
18 |
|
|
3.2 Clinical Efficacy
Budesonide potentiated the treatment efficacy of vitamin AD by 17.5%, with an efficacy of 97.5% in the study group versus that of 80.0% in the control group (P<0.05) (Table 2).
Table 2. Clinical Efficacy
Groups |
n |
Cured |
Markedly Effective |
Effective |
Ineffective |
Total Effective Rate |
Study group |
40 |
20 |
12 |
7 |
1 |
39 |
Control group |
40 |
14 |
9 |
9 |
8 |
32 |
χ2 |
|
|
|
|
|
6.135 |
P |
|
|
|
|
|
0.013 |
3.3 Serum IgE and CPR Levels
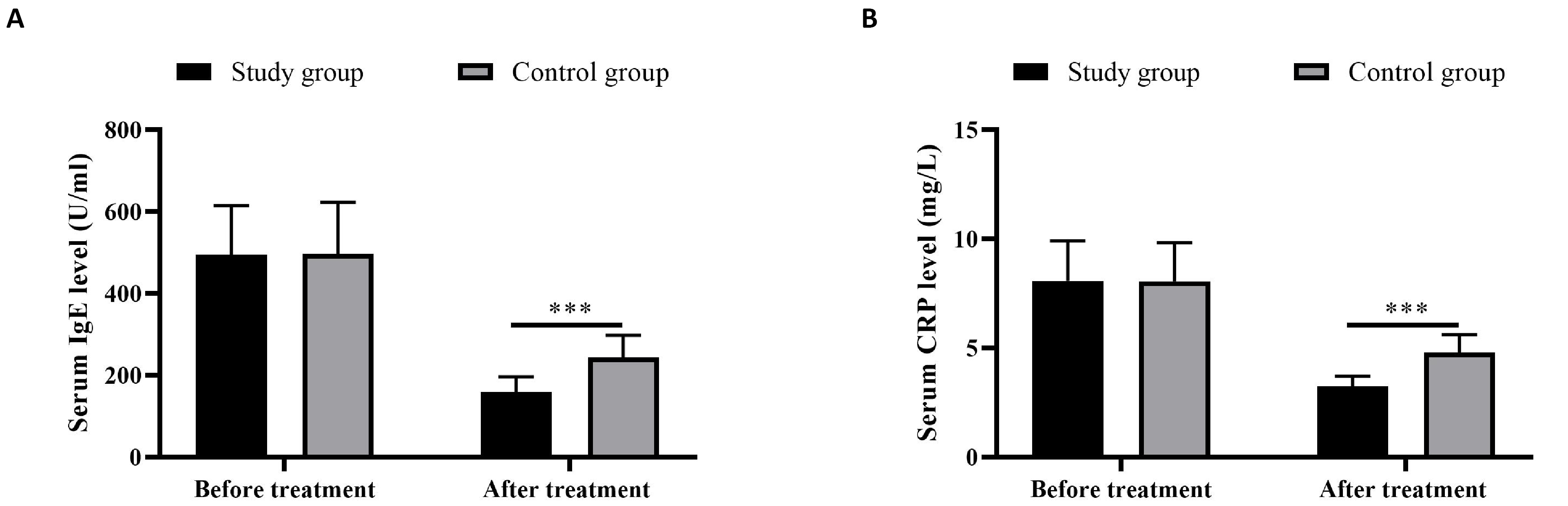
Patients receiving the combined therapy exhibited more reduction in the serum concentrations of IgE and CRP versus those with monotherapy of vitamin AD (P<0.05) (Figure 1).
|
Figure 1. Serum IgE (A) and CPR (B) levels. *** indicated P<0.001.
3.4 Bronchial Asthma Daytime and Night Symptom Scores
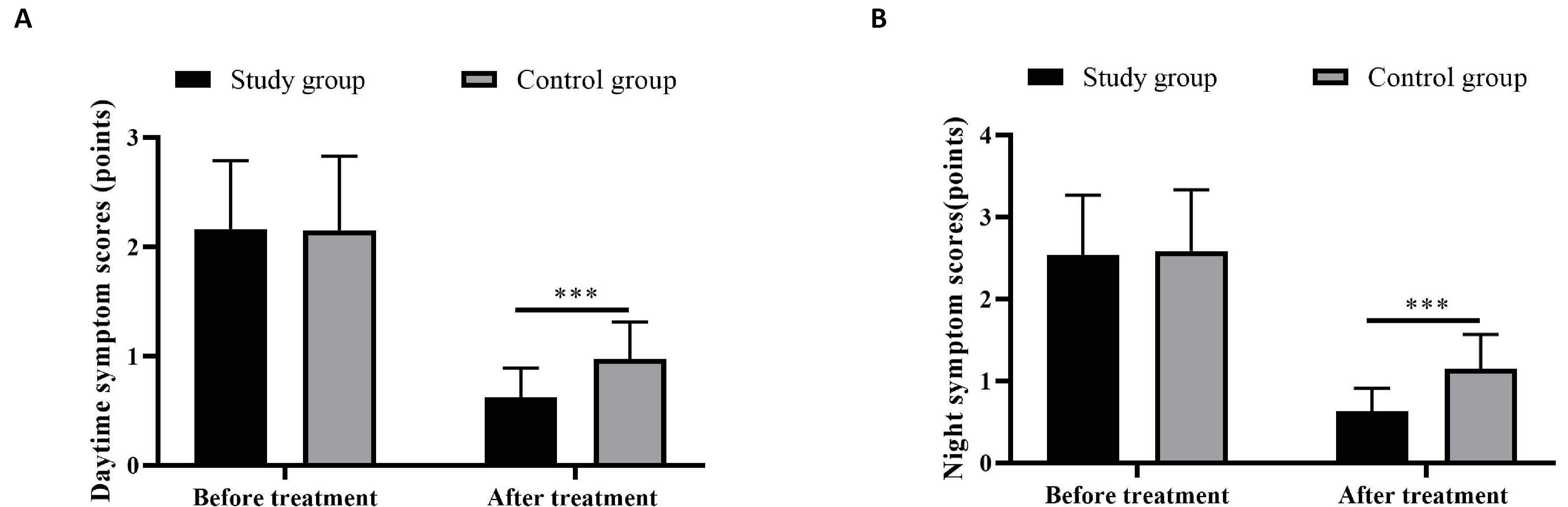
Patients with combined therapy showed lower bronchial asthma symptom scores than those in the control group, suggesting more symptom mitigation produced by budesonide plus vitamin AD (P<0.05) (Figure 2).
|
Figure 2. Bronchial asthma daytime (A) and night symptom (B) scores. *** indicated P< 0.001.
3.5 Pulmonary Function
Budesonide plus vitamin AD offered better lung function restoration for patients versus vitamin AD alone, as evinced by the higher levels of PEF, FVC, and FEV1 (P<0.05) (Table 3).
Table 3. Pulmonary Function (mean±SD)
|
n |
PEF (L/s) |
FVC (L) |
FEV1 (L) |
|||
Before |
After |
Before |
After |
Before |
After |
||
Control Group |
40 |
||||||
Study Group |
40 |
||||||
t |
|
0.1383 |
2.368 |
1.179 |
5.553 |
0.339 |
2.714 |
P |
|
0.890 |
0.020 |
0.242 |
<0.001 |
0.736 |
0.008 |
3.6 Duration of Symptoms
The study group had a shorter duration of fever, cough, shortness of breath, and lung rales than the control group (P<0.05) (Table 4).
Table 4. Symptoms Disappeartime (mean±SD, d)
Groups |
n |
Fever |
Coughing |
Shortness of Breath |
Lung Rales |
Study group |
40 |
2.88±0.31 |
4.06±0.48 |
3.49±0.36 |
3.28±0.41 |
Control group |
40 |
4.12±0.48 |
5.30±0.56 |
4.83±0.49 |
4.72±0.46 |
t |
|
13.725 |
10.633 |
13.938 |
14.780 |
P |
|
<0.05 |
<0.05 |
<0.05 |
<0.05 |
4 DISCUSSION
Bronchial asthma is a common chronic respiratory disease affecting children's health, and its prevalence is increasing year by year worldwide. Bronchial asthma is a chronic inflammatory disease of the entangled airway with multiple cytokines, which include neutrophils, eosinophils, and mast cells. Chronic inflammation could elicit a high airway reaction and airway remodeling, leading to multiple episodes of reversible airflow limitation, cough, shortness of breath, and chest tightness[8-10]. Bronchial asthma is triggered by genetic and environmental factors and features a high prevalence[11-13] in young children.
In the present study, Budesonide potentiated the treatment efficacy of vitamin AD by 17.5%, and patients receiving the combined therapy exhibited more reduction in the serum concentrations of IgE and CRP versus those with monotherapy of vitamin AD, which was in line with the results of previous studies[14-16], in which budesonide with vitamin AD reduced the serum IgE and CRP levels in children with bronchial asthma. Patients with combined therapy showed lower bronchial asthma symptom scores versus the controls, suggesting more symptom mitigation produced by budesonide plus vitamin AD. At present, the management of acute exacerbation of bronchial asthma mainly focuses on asthma relief, anti-inflammation, and clinical symptom mitigation[17]. Glucocorticoids have been reported to be effective in preventing recurrent asthma attacks[18,19]. As an inhaled glucocorticoid, budesonide provides marked mitigation of local inflammation. Vitamin A is an antioxidant that regulates immunity, maintains the integrity of airway mucosa epithelium, promotes normal development of airway smooth muscle, reduces airway hyperresponsiveness, and prevents airway remodeling[20-22]. Studies have demonstrated that vitamin D was associated with airway inflammation in asthma, as vitamin D deficiency leads to an increased frequency of asthma onset in children[23,24]. Feng et al.[25] stated that budesonide combined with vitamin AD was efficacious in the treatment of bronchial asthma. A study by Deng et al.[26] revealed that budesonide could significantly restore lung function in children with bronchial asthma.
Here, budesonide plus vitamin AD offered better lung function restoration for patients versus vitamin AD alone, as evinced by the higher levels of PEF, FVC, and FEV1, and patients in the study group had a shorter duration of symptoms versus controls. Vitamin D is an essential fat-soluble vitamin whose biologically active metabolite is l25(OH)2D3, which exerts its main action through the vitamin D receptor (VDR). It has been found that VDR is present in almost all tissues and cells of the body, including the immune system, lungs, skin, placenta, heart, and brain. Therefore, in addition to regulating calcium and phosphorus balance, vitamin D also has immunomodulatory effects. Vitamin D has been shown to regulate immune functions including the ability to induce immune tolerance in dendritic cells, signaling of activated T cells, development of CD4+, CD25+, Foxp3+, and regulatory T cells, and regulation of tolerance and anti-inflammatory cytokines such as interleukin 10. The present study used budesonide in combination with vitamin AD to improve vitamin A and D levels in patients to regulate disease onset and progression.
Yiqi Wenyang Huwei Tang is composed of Guizhi Tang, Yuping Fengsan, Xianmao and Xian Lingpi. The whole formula has the effect of warming Yang, benefiting Qi, harmonizing Ying and Wei, and invigorating the true essence, enabling the source of Wei-Qi. Long-term Wei-Qi deficiency will cause Yang deficiency, and Wei-Yang deficiency may aggravate the symptoms of fear of wind and cold, and even aversion to cold. Thus, it is necessary to add Paofuzi to protect Wei-Yang and increase the dosage of Baishao. If spleen-Yang deficiency leads to fatigue and obvious shortness of breath, Buzhong Yiqi Tang could be incorporated, while those with spleen-Yang deficiency with loose stools or even edema could be given Shipi Yin. JinKui Shen Qi Pill could be adopted for patients with insufficient kidney Qi and abnormal breathing. Liuwei Dihuang Pills are available for patients with Yin deficiency, dizziness, backache, tinnitus, and less tongue coating.
However, there were some limitations in this study. The short duration of the study and the small sample size may cause selection bias and possible random error. This study only observed and followed up with patients for a short period of time and did not implement long-term follow-up to observe the efficacy of the treatment. Only IgE and CRP, which trigger asthma attacks, have been tested and analysed, while studies on other inflammatory factors and other triggers of asthma are lacking.
5 CONCLUSION
Budesonide plus vitamin AD yields promising clinical effects on the treatment of bronchial asthma by alleviating the clinical symptoms, improving the pulmonary function of children, and lowering the levels of serum IgE and CRP. Furthermore, traditional Chinese medicine possesses unique merits in the management of bronchial asthma, especially in the remission stage of bronchial asthma, with less toxic side effects.
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declared no conflict of interest.
Author Contribution
Wang Y and Tan Y designed this study and wrote the article; Zhang L, Zheng L and Cui Y collected the data and performed the statistical analysis; Han L, Xie J, Zhang M and An X revised the papers for important intellectual content; all authors approved the final version.
Abbreviation List
CRP, C-reactive protein
FEV1, Forced expiratory volume in one second
FVC, Forced vital capacity
PEF, Peak expiratory flow
References
[1] Hughes TE, Stansfield L, Kumar P et al. A prospective evaluation on the interaction of fluconazole and voriconazole on serum concentrations of budesonide in patients treated for gastrointestinal GVHD. Bone Marrow Transplant, 2020; 55: 1085-1092. DOI: 10.1038/s41409-020-0786-8
[2] Kewcharoen J, Mekraksakit P, Limpruttidham N et al. Budesonide for protein losing enteropathy in patients with Fontan circulation: a systematic review and meta-analysis. World J Pediatr Congenit Heart Surg, 2020; 11: 85-91. DOI: 10.1177/2150135119872196
[3] Lucendo AJ. Pharmacological treatments for eosinophilic esophagitis: current options and emerging therapies. Expert Rev Clin Immunol, 2020; 16: 63-77. DOI: 10.1080/1744666X.2019.1705784
[4] Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev, 2018; 9: CD001506. DOI: 10.1002/14651858.CD001506.pub4
[5] Liu L, Yue D, Hu L et al. Relationship between interleukin-13 rs20541 single nucleotide polymorphisms and therapeutic efficacy in children with asthma. J Int Med Res, 2020; 48: 300060520929179. DOI: 10.1177/0300060520929179
[6] Šimková K, Joost B, Imanidis G. Production of fast-dissolving low-density powders for improved lung deposition by spray drying of a nanosuspension. Eur J Pharm Biopharm, 2020; 146: 19-31. DOI: 10.1016/j.ejpb.2019.11.003
[7] Cloutier MM, Dixon AE, Krishnan JA et al. Managing Asthma in Adolescents and Adults: 2020 Asthma Guideline Update From the National Asthma Education and Prevention Program. JAMA, 2020; 324: 2301-2317. DOI: 10.1001/jama.2020.21974
[8] Li H, Lin Y, Ye Q et al. Airway inflammation and remodeling of cigarette smoking exposure ovalbumin-induced asthma is alleviated by CpG oligodeoxynucleotides via affecting dendritic cell-mediated Th17 polarization. Int Immunopharmacol, 2020; 82: 106361. DOI: 10.1016/j.intimp.2020.106361
[9] Zhang Y, Wang H. Efficacy of montelukast sodium chewable tablets combined with inhaled budesonide in treating pediatric asthma and its effect on inflammatory factors. Pharmazie, 2019; 74: 694-697. DOI: 10.1691/ph.2019.9582
[10] Antonino RSCMQ, Nascimento TL, Junior ERO et al. Thermoreversible mucoadhesive polymer-drug dispersion for sustained local delivery of budesonide to treat inflammatory disorders of the GI tract. J Control Release, 2019; 303: 12-23. DOI: 10.1016/j.jconrel.2019.04.011
[11] Wagstaff KM, Headey S, Telwatte S et al. Molecular dissection of an inhibitor targeting the HIV integrase dependent preintegration complex nuclear import. Cell Microbiol, 2019; 21: e12953. DOI: 10.1111/cmi.12953
[12] Kunc P, Fabry J, Lucanska M et al. Biomarkers of Bronchial Asthma. Physiol Res, 2020; 69(Suppl 1): S29-S34. DOI: 10.33549/physiolres.934398
[13] Salice M, Rizzello F, Calabrese C et al. A current overview of corticosteroid use in active ulcerative colitis. Expert Rev Gastroenterol Hepatol, 2019; 13: 557-561. DOI: 10.1080/17474124.2019.1604219
[14] Ha T, Valentine R, Moratti S et al. The efficacy of a novel budesonide chitosan gel on wound healing following endoscopic sinus surgery. Int Forum Allergy Rhinol, 2018; 8: 435-443. DOI: 10.1002/alr.22057
[15] Li L, Yang C, Feng X et al. Effects of intratracheal budesonide during early postnatal life on lung maturity of premature fetal rabbits. Pediatr Pulmonol, 2018; 53: 28-35. DOI: 10.1002/ppul.23889
[16] Dubey NK, Jain P, Bedi S. Development and Validation of Total Levothyroxine and Total Liothyronine in Human Serum using Chemiluminescence Micro Particle Immunoassay and Its Application to Bioequivalence Study. J Mod Pharmacol Pathol, 2023; 1: 3. DOI: 10.53964/jmpp.2023003
[17] Li S, Xie A, Li H et al. A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. J Control Release, 2019; 316: 66-78. DOI: 10.1016/j.jconrel.2019.10.054
[18] Jeon HH, Lee HJ, Jang HW et al. Clinical outcomes and predictive factors in oral corticosteroid-refractory active ulcerative colitis. World J Gastroenterol, 2013; 19: 265-73. DOI: 10.3748/wjg.v19.i2.265
[19] Li H, Chen Z, Lin Y et al. CpG-ODNs and budesonide act synergistically to improve allergic responses in combined allergic rhinitis and asthma syndrome induced by chronic exposure to ovalbumin by modulating the TSLP-DC-OX40L axis. Inflammation, 2018; 41: 1304-1320. DOI: 10.1007/s10753-018-0779-6
[20] Reddel HK. Updated Australian guidelines for mild asthma: what's changed and why? Aust Prescr, 2020; 43: 220-224. DOI: 10.1016/j.jconrel.2019.04.011
[21] O'Byrne PM, FitzGerald JM, Bateman ED et al. Inhaled combined budesonide–formoterol as needed in mild asthma. N Engl J Med, 2018; 378: 1865-1876. DOI: 10.1056/NEJMoa1715274
[22] Leng D, Thanki K, Fattal E et al. Engineering of budesonide-loaded lipid-polymer hybrid nanoparticles using a quality-by-design approach. Int J Pharm, 2018; 548: 740-746. DOI: 10.1016/j.ijpharm.2017.08.094
[23] Busby J, Murray L, Mills K et al. A combined connectivity mapping and pharmacoepidemiology approach to identify existing medications with breast cancer causing or preventing properties. Pharmacoepidemiol Drug Saf, 2018; 27: 78-86. DOI: 10.1002/pds.4345
[24] Reed CC, Safta AM, Qasem S et al. Combined and alternating topical steroids and food elimination diet for the treatment of eosinophilic esophagitis. Dig Dis Sci, 2018; 63: 2381-2388. DOI: 10.1007/s10620-018-4931-9
[25] Feng J, Ding G, Xie Y, et al. Efficacy of budesonide/formoterol and tiotropium combination for the treatment of Chinese patients with chronic obstructive pulmonary disease. Medicine (Baltimore), 2018; 97: e10841. DOI: 10.1097/MD.0000000000010841
[26] Deng J, Chen F, Lai Y et al. Lack of additional effects of long-term, low-dose clarithromycin combined treatment compared with topical steroids alone for chronic rhinosinusitis in China: a randomized, controlled trial. Int Forum Allergy Rhinol, 2018; 8: 8-14. DOI: 10.1002/alr.22041
Copyright © 2023 The Author(s). This open-access article is licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, sharing, adaptation, distribution, and reproduction in any medium, provided the original work is properly cited.




 Copyright ©
Copyright ©